|
※サムネイル画像をクリックすると拡大画像が表示されます。
|
|
|
| 別品名 |
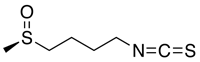
L-Sulforaphane, 1-Isothiocyanato-4-(methylsulfinyl)-butane
|
| 別包装 |
あり
|
| 純度 |
>=98%
|
| 分子量 |
177.29
|
| CAS RN® |
142825-10-3
|
| 構造 |
C6H11NOS2
|
| 溶解性 |
Soluble in ethanol, methanol, or ethyl acetate. Miscible with water and/or DMSO.
|
| 使用目的 |
R-Sulforaphane is a naturally-occurring isothiocyanate found broccoli. This compound exhibits antioxidative, anticancer, and chemopreventive activities; research suggests that it may be more bioactive than the S-isomer. R-Sulforaphane increases expression of phase II enzymes such as glutathione-S-transferase and quinone reductase; it also increases activity and expression of glucuronosyl transferase and epoxide hydrolase. At low doses, R-sulforaphane promotes proliferation of stem cells and hematopoiesis. This compound also inhibits the aryl hydrocarbon receptor.Please note: This compound is a neat liquid at room temperature, clear to slightly yellow in color. It is not dissolved in solvent. In small quantities the material will coat the sides of the ampoule it is shipped in and may appear to be invisible, or it may be trapped in the tip of the ampoule. Wash the upper and lower parts of the ampoule with solvent to ensure all of the material is obtained.
|
| 概要 |
Naturally occuring isothiocyanate found in broccoli; AhR antagonist.
|
| その他 |
[外観]Very slight yellowish liquid
|
| 参考文献 |
Abdull Razis AF, Hanlon N, Soltys E, et al. The naturally occurring aliphatic isothiocyanates sulforaphane and erucin are weak agonists but potent non-competitive antagonists of the aryl hydrocarbon receptor. Arch Toxicol. 2012 Oct;86(10):1505-14. PMID: 22643862.Zanichelli F, Capasso S, Cipollaro M, et al. Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age (Dordr). 2012 Apr;34(2):281-93. PMID: 21465338.Abdull Razis AF, Bagatta M, De Nicola GR, et al. Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: importance of side-chain substituent and chirality. Arch Toxicol. 2011 Aug;85(8):919-27. PMID: 21132492.Abdull Razis AF, Iori R, Ioannides C. The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Int J Cancer. 2011 Jun 15;128(12):2775-82. PMID: 20726001.Christine A. Houghton, Sulforaphane: Its ''Coming of Age'' as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Medicine and Cellular Longevity Volume 2019, Article ID 2716870, 27 pages https://doi.org/10.1155/2019/2716870
|
|
| メーカー |
品番 |
包装 |
|
LKT
|
S8046
|
25 MG
|
※表示価格について
| 当社在庫 |
なし
|
| 納期目安 |
1週間程度
|
| 保存温度 |
-20℃
|
|
※当社では商品情報の適切な管理に努めておりますが、表示される法規制情報は最新でない可能性があります。
また法規制情報の表示が無いものは、必ずしも法規制に非該当であることを示すものではありません。
商品のお届け前に最新の製品法規制情報をお求めの際はこちらへお問い合わせください。
|
※当社取り扱いの試薬・機器製品および受託サービス・創薬支援サービス(納品物、解析データ等)は、研究用としてのみ販売しております。
人や動物の医療用・臨床診断用・食品用としては、使用しないように、十分ご注意ください。
法規制欄に体外診断用医薬品と記載のものは除きます。
|
|
※リンク先での文献等のダウンロードに際しましては、掲載元の規約遵守をお願いします。
|
|
※CAS Registry Numbers have not been verified by CAS and may be inaccurate.
|