|
※サムネイル画像をクリックすると拡大画像が表示されます。
|
|
|
| 別品名 |
D,L-Sulforaphane, Sulforaphane, 4-(Methylsulfinyl)-butyl isothiocyanate??, 1-Isothiocyanato-4-(methylsulfinyl)-butane
|
| 別包装 |
あり
|
| 由来詳細 |
Synthetic racemic mixture of R and S isomers
|
| 純度 |
>=99%
|
| 分子量 |
177.29
|
| CAS RN® |
4478-93-7
|
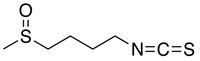
| 構造 |
C6H11NOS2
|
| 溶解性 |
Soluble in ethanol, methanol, or ethyl acetate. Miscible with water and/or DMSO.
|
| 使用目的 |
R,S-Sulforaphane is a synthetic isothiocyanate. R-Sulforaphane is the naturally-occurring isomer, found in broccoli. This compound exhibits antioxidative, neuroprotective, anti-inflammatory, anti-atherosclerotic, neuromodulatory, cardioprotective, anticoagulant, antibiotic, anticancer chemotherapeutic, and chemopreventive activities; research suggests that the R-isomer may be more bioactive than the S-isomer. R,S-Sulforaphane induces activity and expression of phase II enzymes, inhibits the aryl hydrocarbon receptor, and downregulates expression of HDACs and STAT5. In cellular and animal models of melanoma, this compound inhibits tumor growth and cell proliferation. R,S-Sulforaphane also induces autophagy. In animal models of Alzheimer’s disease, R,S-sulforaphane lessens cognitive impairment. Additionally, this compound increases activity of Nrf2 and expression of catalase, protecting against UV-induced oxidative damage in ex vivo models. In other animal models, R,S-sulforaphane inhibits TNF-α-induced adhesion of monocytes to epithelial cells and decreases levels of VCAM-1. In myocytes, this compound prevents angiotensin II-induced hypertrophy. Additionally, R,S-sulforaphane inhibits platelet aggregation by suppressing PI3K/Akt signaling. R,S-Sulforaphane also displays antibacterial efficacy against Escherichia. Please note: This compound is a neat liquid at room temperature, clear to slightly yellow in color. It is not dissolved in solvent. In small quantities the material will coat the sides of the ampoule it is shipped in and may appear to be invisible, or it may be trapped in the tip of the ampule. Wash the upper and lower parts of the ampoule with solvent to ensure all of the material is obtained.
|
| 概要 |
Phase II enzyme inducer; Nrf2 activator; antioxidant with anticarcinogenic properties.
|
| その他 |
[外観]Slight yellowish liquid
|
| 参考文献 |
Lee JH, Jeong JK, Park SY. Sulforaphane-induced autophagy flux prevents prion protein-mediated neurotoxicity through AMPK pathway. Neuroscience. 2014 Oct 10;278:31-9. PMID: 25130556.Zhang R, Zhang J, Fang L, et al. Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer's disease-like lesions. Int J Mol Sci. 2014 Aug 18;15(8):14396-410. PMID: 25196440.Nallasamy P, Si H, Babu PV, et al. Sulforaphane reduces vascular inflammation in mice and prevents TNF-α-induced monocyte adhesion to primary endothelial cells through interfering with the NF-κB pathway. J Nutr Biochem. 2014 Aug;25(8):824-33. PMID: 24880493.Pinz S, Unser S, Rascle A. The natural chemopreventive agent sulforaphane inhibits STAT5 activity. PLoS One. 2014 Jun 9;9(6):e99391. PMID: 24910998.Wu QQ, Zong J, Gao L, et al. Sulforaphane protects H9c2 cardiomyocytes from angiotensin II-induced hypertrophy. Herz. 2014 May;39(3):390-6. PMID: 23784363.Kleszczy?ski K, Ernst IM, Wagner AE, et al. Sulforaphane and phenylethyl isothiocyanate protect human skin against UVR-induced oxidative stress and apoptosis: role of Nrf2-dependent gene expression and antioxidant enzymes. Pharmacol Res. 2013 Dec;78:28-40. PMID: 24121007.Chuang WY, Kung PH, Kuo CY, et al. Sulforaphane prevents human platelet aggregation through inhibiting the phosphatidylinositol 3-kinase/Akt pathway. Thromb Haemost. 2013 Jun;109(6):1120-30. PMID: 23426129.Thakkar A, Sutaria D, Grandhi BK, et al. The molecular mechanism of action of aspirin, curcumin and sulforaphane combinations in the chemoprevention of pancreatic cancer. Oncol Rep. 2013 Apr;29(4):1671-7. PMID: 23404329.Abdull Razis AF, Hanlon N, Soltys E, et al. The naturally occurring aliphatic isothiocyanates sulforaphane and erucin are weak agonists but potent non-competitive antagonists of the aryl hydrocarbon receptor. Arch Toxicol. 2012 Oct;86(10):1505-14. PMID: 22643862.Wu HZ, Fei HJ, Zhao YL, et al. Antibacterial mechanism of sulforaphane on Escherichia coli. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012 May;43(3):386-90. PMID: 22812243.Do DP, Pai SB, Rizvi SA, et al. Development of sulforaphane-encapsulated microspheres for cancer epigenetic therapy. Int J Pharm. 2010 Feb 15;386(1-2):114-21. PMID: 19922783.Christine A. Houghton, Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Medicine and Cellular Longevity Volume 2019, Article ID 2716870, 27 pages https://doi.org/10.1155/2019/2716870
|
|
| メーカー |
品番 |
包装 |
|
LKT
|
S8044
|
100 MG
|
※表示価格について
| 当社在庫 |
なし
|
| 納期目安 |
1週間程度
|
| 法規制 |
危
|
| 保存温度 |
-20℃
|
|