|
※サムネイル画像をクリックすると拡大画像が表示されます。
|
|
|
| 適用 |
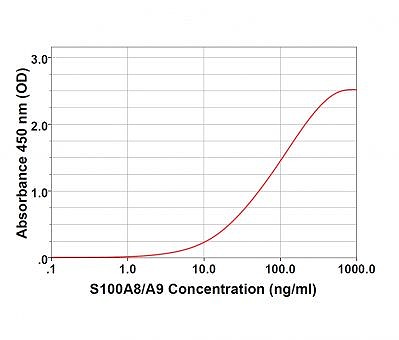
Enzyme Linked Immunosorbent Assay
|
| 免疫動物 |
Mouse
|
| その他 |
[活性・反応性(特記事項)]サンドイッチ法[内容・その他(情報)]
Alternative names
MRP8: S100A8, Calgranulin A, CP-10 (in mouse)
MRP14: S100A9, Calgranulin B
MRP8/14: Calprotectin, L1, (p8,14), p34
Migration inhibitory factor-related protein (MRP) -8 and -14 belong to the S-100 family of calcium binding proteins associated with myeloid cell differentiation. They are highly expressed in resting neutrophils keratinocytes (particularly in psoriasis), in infiltrating tissue macrophages and onepithelial cells in active inflammatory disease. The heterogeneity of macrophage subpopulations in chronic or acute inflammation is reflected by different expression of MRP8 and MRP14. Phagocytes expressing MRP8 and MRP14 belong to the early infiltrating cells, while MRP8 aloneis found in chronic inflammatory tissues. The partially antagonistic functions of MRP8, MRP14 and of the Ca2+-dependent MRP8/14 heterocomplex makes them versatile mediators.
Functions
One major function of the MRP8/14heterocomplex is its antimicrobial activity (hence the name calprotectin). MRP8/14 inhibits the growth of pathogens through competition for zinc. MRP8/14 also induces apoptosis of certain tumor cells. These activities are abrogated by Zn2+ and otherdivalent cations, but not byCa2+ or Mg2+.
Another important property specific for the MRP8/14 heterocomplex is its unique role as a fatty acid transport protein. The Ca2+-dependent fatty acid-MRP8/14 complex is the major carrier of polyunsaturated fatty acids in neutrophils.The complex is expressed in resting cells and moves to the membrane upon stimulation. Zn2+ inhibits the fatty acid carrier capacity of MRP8/14 already at physiological Zn2+ serum concentrations, so that fatty acids are not carried in the blood circulation. This makes MRP8/14 an important mediator between calcium signaling and arachidonic acid effects.
MRP8 (and MRP8/14, but not MRP14 alone) is secreted upon stimulation with inflammatory mediators. It is a potent chemoattractant for neutrophilsandmonocytes. However, MRP8 does not increase intracellular Ca2+ nor evoke an oxidative burst and granular enzyme release like e.g. C5a. Exposure of MRP8 to hypochlorite, possibly generated by activated neutrophils, convertsit to the inactive disulfide-linked dimer. Glucocorticoids up-regulate induction of MRP8 by inflammatory mediators. MRP8 may contribute to the regulation of fetal-maternal interactions, which would explain why inactivation of the MRP8 gene in the mouse is embryonic lethal.
Thelack of co-expression of MRP14 with MRP8 in activated macrophages points to their different roles. The C-terminal sequence of MRP14 is identical to the N-terminus of neutrophil immobilizing factors. MRP14 can be phosphorylated which increases its Ca2+-binding capacity. It then tends to move from the cytosol to membranes and the cytoskeleton. MRP14 has been shown to be associated with a subpopulation of neutrophils with metastasis-enhancing abilities.
Biochemistry
Human MRP8 has a molecularweight of 11.0kD, while human MRP14 exists in a 13.3kD and a truncated 12.9kD form. Ca2+ induces the formation of heterocomplexes of the form (MRP8)(MRP14) (abbreviated MRP8/14), (MRP8)2(MRP14), and (MRP8/14)2. There are two EF-hand motifs each on MRP8and MRP14. MRP14 shows a higher affinity for calcium than MRP8, and the affinity of the C-terminal EF2 is higher than that of the N-terminal EF1. The C-terminal domain also mainly determines the specificity of dimerization.The helix in EF2 undergoesa large conformational change upon calcium binding and may play a role as a trigger for Ca2+ induced conformational change.
The antimicrobial activity of MRP8/14 is caused by zinc chelation by a polyhistidine sequence nearthe C-terminus of
|
|
| メーカー |
品番 |
包装 |
|
BMA
|
S-1011
|
1*96 TEST
|
※表示価格について
| 当社在庫 |
なし
|
| 納期目安 |
1週間程度
|
| 法規制 |
安・危
|
| 保存温度 |
4℃
|
|
※当社では商品情報の適切な管理に努めておりますが、表示される法規制情報は最新でない可能性があります。
また法規制情報の表示が無いものは、必ずしも法規制に非該当であることを示すものではありません。
商品のお届け前に最新の製品法規制情報をお求めの際はこちらへお問い合わせください。
|
※当社取り扱いの試薬・機器製品および受託サービス・創薬支援サービス(納品物、解析データ等)は、研究用としてのみ販売しております。
人や動物の医療用・臨床診断用・食品用としては、使用しないように、十分ご注意ください。
法規制欄に体外診断用医薬品と記載のものは除きます。
|
|
※リンク先での文献等のダウンロードに際しましては、掲載元の規約遵守をお願いします。
|
|
※CAS Registry Numbers have not been verified by CAS and may be inaccurate.
|