|
※サムネイル画像をクリックすると拡大画像が表示されます。
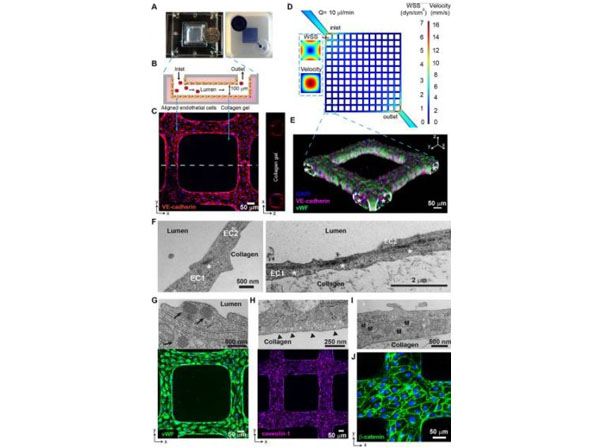
Characterization of 3D brain microvessels. (A) (Left) Photo of an assembled 3D microvessel device with a dime (diameter, 17.9?mm). (Right) 3D microvessels perfused with food dye. (B) Schematic cross-sectional view of the 3D microvessels. (C) Immunofluorescence assay (IFA) z-projection of confocal sections of a 3D brain microvessel (left) and cross-sectional view (right) labeled with anti-VE-cadherin (red) and DAPI (blue). (D) Mid-plane flow velocity (z?=?50?μm) and estimated WSS (z?=?0?μm) distributions in the grid geometry, simulated with COMSOL prior to collagen remodeling by HBMEC (see Materials and Methods). Inlaid cross-sectional views represent the lumen at the first branch after the inlet. (E) 3D reconstruction of a grid portion. Colors indicate anti-VE-cadherin antibody (red), anti-VWF antibody (green), and DAPI (blue). Asterisk, lumen. (F) Transmission electron microscopy (TEM) showing endothelial junctions and focal contacts. EC1 and EC2, endothelial cells 1 and 2; asterisk, electron-dense contacts. (G) TEM showing Weibel-Palade bodies (arrows, top) and IFA z-projection of VWF (green, bottom). (H) TEM image of polarized caveolae (arrowheads, top) and IFA z-projection of caveolin-1 (magenta, bottom). (I) TEM image reveals high mitochondrial (M) content of HBMEC. (J) IFA z-projection of adherens junctions stained with anti-β-catenin antibody (green). Nuclei in panels G, H, and J were stained with DAPI (blue). Rat tail (p/n RT-T297). Fig 1. PMID: 31138740.
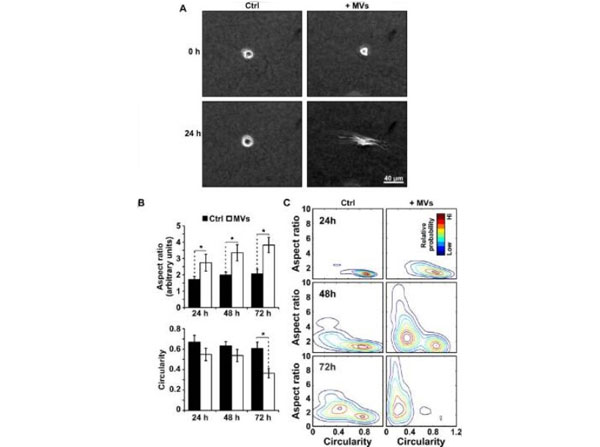
MVs induce morphological changes in epithelial cells cultured in 3D (A) Phase contrast images at 0 h and 24 h of MCF10a cells supplemented with MVs (+MVs) or culture media alone (Ctrl). (B) Corresponding quantification of aspect ratio and (C) circularity shape parameters measured from at least 60 cells from 3 independent experiments. Mean ± SE (D) Cell shape population distributions plotted as a function of the aspect ratio to circularity shape parameters. *p<0.05. Rat tail (p/n RT-T297). Fig 2. PMID: 26477404.
3D ECM remodeling by carcinoma-derived fibroblasts. (A) 3D reconstruction from confocal reflectance sections of a spheroid (S) composed of carcinoma-derived primary fibroblasts invading into a 3D collagen scaffolds. Cells were allowed to invade into the ECM for a period of 48 h. The original spheroid boundary is indicated by the dashed line. (B) Phase contrast image of the invading cells (indicated by the asterisks) and the corresponding confocal reflectance image showing the collagen remodeling perpendicular to the spheroid boundary (arrow). (C) Confocal reflectance images of collagen showing a Z stack of the microtrack left in the wake of a single cell migration due to ECM remodeling and (D) the corresponding 3D reconstruction of the microtrack. Rat tail (p/n RT-T297). Fig 4. PMID: 25866589

|

|
|
Characterization of 3D brain microvessels. (A) (Left) Photo of an assembled 3D microvessel device with a dime (diameter, 17.9?mm). (Right) 3D microvessels perfused with food dye. (B) Schematic cross-sectional view of the 3D microvessels. (C) Immunofluorescence assay (IFA) z-projection of confocal sections of a 3D brain microvessel (left) and cross-sectional view (right) labeled with anti-VE-cadherin (red) and DAPI (blue). (D) Mid-plane flow velocity (z?=?50?μm) and estimated WSS (z?=?0?μm) distributions in the grid geometry, simulated with COMSOL prior to collagen remodeling by HBMEC (see Materials and Methods). Inlaid cross-sectional views represent the lumen at the first branch after the inlet. (E) 3D reconstruction of a grid portion. Colors indicate anti-VE-cadherin antibody (red), anti-VWF antibody (green), and DAPI (blue). Asterisk, lumen. (F) Transmission electron microscopy (TEM) showing endothelial junctions and focal contacts. EC1 and EC2, endothelial cells 1 and 2; asterisk, electron-dense contacts. (G) TEM showing Weibel-Palade bodies (arrows, top) and IFA z-projection of VWF (green, bottom). (H) TEM image of polarized caveolae (arrowheads, top) and IFA z-projection of caveolin-1 (magenta, bottom). (I) TEM image reveals high mitochondrial (M) content of HBMEC. (J) IFA z-projection of adherens junctions stained with anti-β-catenin antibody (green). Nuclei in panels G, H, and J were stained with DAPI (blue). Rat tail (p/n RT-T297). Fig 1. PMID: 31138740.
|
|
| 別品名 |
Rat tissue, Rat Collagen, Rat Type I Collagen
|
| 種由来 |
Rat
|
| 参考文献 |
[Pub Med ID]31138740
|
|
| メーカー |
品番 |
包装 |
|
RKL
|
RT-T297
|
50 PACK
|
※表示価格について
| 当社在庫 |
なし
|
| 納期目安 |
約10日
|
| 保存温度 |
-20℃
|
|
※当社では商品情報の適切な管理に努めておりますが、表示される法規制情報は最新でない可能性があります。
また法規制情報の表示が無いものは、必ずしも法規制に非該当であることを示すものではありません。
商品のお届け前に最新の製品法規制情報をお求めの際はこちらへお問い合わせください。
|
※当社取り扱いの試薬・機器製品および受託サービス・創薬支援サービス(納品物、解析データ等)は、研究用としてのみ販売しております。
人や動物の医療用・臨床診断用・食品用としては、使用しないように、十分ご注意ください。
法規制欄に体外診断用医薬品と記載のものは除きます。
|
|
※リンク先での文献等のダウンロードに際しましては、掲載元の規約遵守をお願いします。
|




